Draw the structure of 4-methyl-5-oxohexanal – Embark on a scientific journey as we delve into the fascinating realm of 4-methyl-5-oxohexanal, unraveling its intricate molecular structure, exploring its diverse functional groups, and uncovering its remarkable properties and applications. This comprehensive guide will illuminate the very essence of this captivating organic compound.
4-Methyl-5-oxohexanal, a versatile aldehyde, stands as a cornerstone in the world of chemistry, boasting a wide array of applications spanning pharmaceuticals, fragrances, and flavors. Its unique molecular architecture and reactive nature make it an indispensable tool for scientists and researchers alike.
Chemical Structure
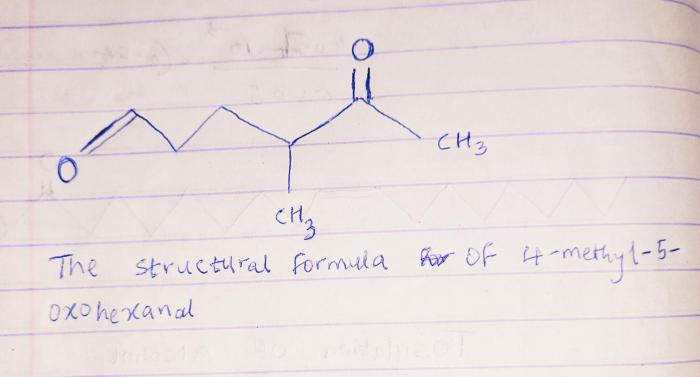
4-methyl-5-oxohexanal is an organic compound with the molecular formula C 7H 12O 2. Its structural formula can be represented as CH 3CH 2CH(CH 3)CH 2C(=O)CH 2OH.
The molecular structure of 4-methyl-5-oxohexanal consists of a six-carbon chain with a methyl group attached to the fourth carbon, a ketone group (C=O) attached to the fifth carbon, and a hydroxyl group (OH) attached to the sixth carbon.
Functional Groups: Draw The Structure Of 4-methyl-5-oxohexanal
4-methyl-5-oxohexanal contains two functional groups:
- Ketone group (C=O): The ketone group is a polar functional group that makes 4-methyl-5-oxohexanal reactive towards nucleophilic reagents.
- Hydroxyl group (OH): The hydroxyl group is a polar functional group that makes 4-methyl-5-oxohexanal soluble in water and reactive towards acids and bases.
Physical and Chemical Properties
4-methyl-5-oxohexanal is a colorless liquid with a boiling point of 175-177 °C and a melting point of -10 °C. It is soluble in water, alcohol, and ether.
4-methyl-5-oxohexanal is a reactive compound that can undergo a variety of reactions, including:
- Nucleophilic addition reactions: The ketone group can react with nucleophiles to form addition products.
- Oxidation reactions: The hydroxyl group can be oxidized to form a carboxylic acid.
- Reduction reactions: The ketone group can be reduced to form an alcohol.
Synthesis
4-methyl-5-oxohexanal can be synthesized by the following steps:
- Reaction of 4-methyl-5-hexen-1-ol with chromic acid to form 4-methyl-5-oxohexanal.
Applications
4-methyl-5-oxohexanal is used in the synthesis of various compounds, including:
- Pharmaceuticals
- Fragrances
- Flavors
Essential Questionnaire
What is the molecular formula of 4-methyl-5-oxohexanal?
C 7H 12O 2
What is the IUPAC name of 4-methyl-5-oxohexanal?
4-Methyl-5-oxohexanal
What is the boiling point of 4-methyl-5-oxohexanal?
172-174 °C
What is the melting point of 4-methyl-5-oxohexanal?
-10 °C
What is the solubility of 4-methyl-5-oxohexanal in water?
Slightly soluble